July 28, 2011, 5:50 pm
The British drug maker AstraZeneca has settled in principle nearly all of the American product liability lawsuits over Seroquel, its blockbuster antipsychotic drug, the company said in a quarterly earnings report on Thursday.
All but 250 of the 28,700 cases have been settled, most with written agreements, AstraZeneca said in the securities filing. It had previously reported settling most of those cases, but the new filing showed how far the company has whittled away at the remaining product liability litigation. Last quarter, it had reported 2,600 outstanding cases.
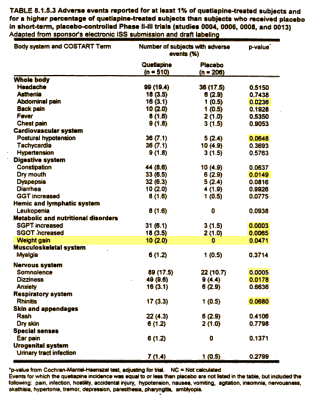
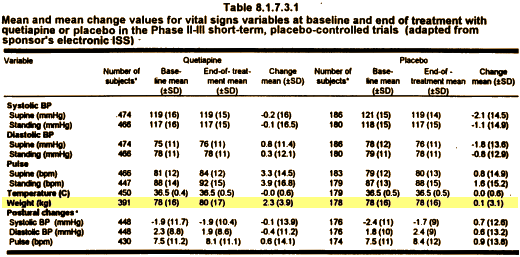
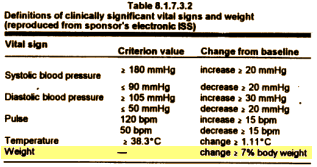
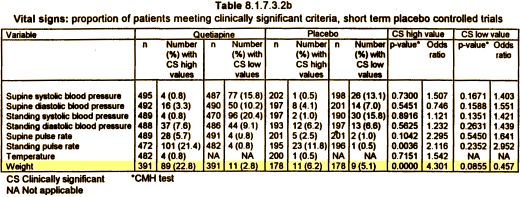
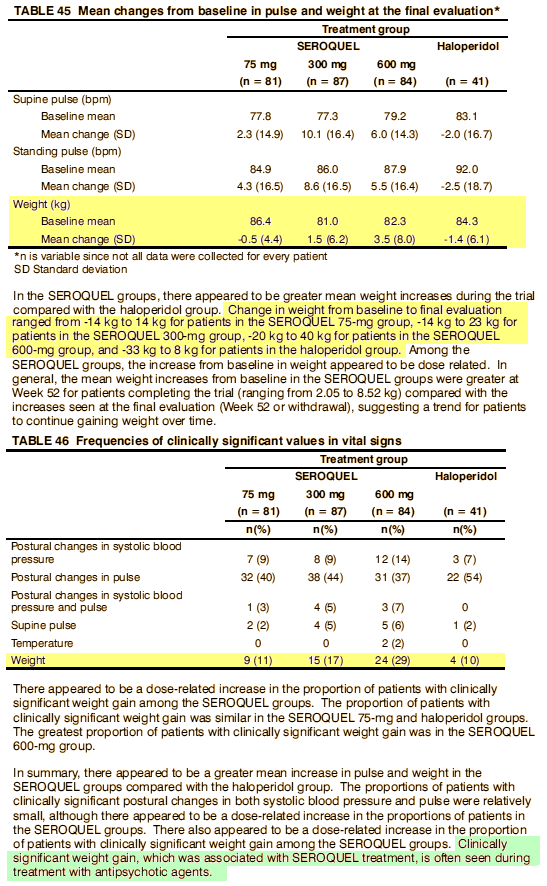
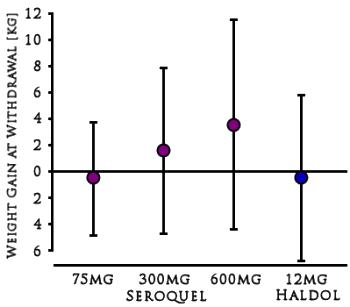
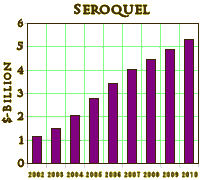
Most of the plaintiffs argued that they had been misled about the risks of diabetes and weight gain caused by Seroquel, the company’s second-best-selling product with $5.3 billion in worldwide sales last year, behind the cholesterol drug Crestor.
In the filing on Thursday, AstraZeneca said it added $55 million last quarter to the previously reported $592 million set-aside, for a total of $647 million to settle the litigation.
“Is it a good move?” said Les Funtleyder, health care analyst for Miller Tabak. “Yes, anytime you can settle these suits, it’s good.”
The set-aside includes the $68.5 million that the drug maker agreed to pay in March to 37 states, settling charges that it had illegally marketed Seroquel.
AstraZeneca also had to pay $520 million last year to settle federal investigations into its marketing of Seroquel.
At that time, Kathleen Sebelius, the secretary of health and human services, had accused the company of paying kickbacks to doctors while promoting the drug for unapproved uses by children, the elderly, veterans and prisoners.
AstraZeneca did not admit to any misconduct, while settling the federal case and signing a corporate integrity agreement, essentially putting it on probation with the government.
The company also said in the filing that its legal fees in Seroquel cases amounted to $743 million, partly covered by insurance. That figure has risen from $688 million estimated a year ago.
If the estimates hold, AstraZeneca will have paid a total of about $1.9 billion to defend and settle the personal injury cases and government investigations.
The figure represents less than five months of Seroquel sales.
That is far less than Eli Lilly paid to settle similar charges regarding its antipsychotic drug Zyprexa. Lilly paid more than $1.2 billion to settle lawsuits in 2007 and $1.4 billion to the government in 2009, including a criminal fine of $515 million.
At the time, that fine was the largest ever for a United States corporation. It has since eclipsed by the $1.3 billion criminal fine Pfizer paid as part of a $2.3 billion settlement of charges it had illegally marketed the painkiller Bextra and other drugs.
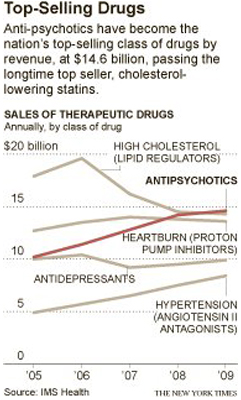
Drug makers have become the biggest targets of government antifraud investigations.
Legal discovery in the Seroquel cases has provided some of the most embarrassing or damaging disclosures over AstraZeneca’s past research and marketing practices, including a 1997 memo praising the company’s work to put a “positive spin” on a “cursed study” and highlighting one official who “has done a great ‘smoke-and-mirrors’ job!”
Another internal e-mail unsealed in court said AstraZeneca had “buried” unfavorable studies. A publications manager for the company wrote, “the larger issue is how we face the outside world when they begin to criticize us for suppressing data.”
The British drug maker AstraZeneca has settled in principle nearly all of the American product liability lawsuits over Seroquel, its blockbuster antipsychotic drug, the company said in a quarterly earnings report on Thursday.
All but 250 of the 28,700 cases have been settled, most with written agreements, AstraZeneca said in the securities filing. It had previously reported settling most of those cases, but the new filing showed how far the company has whittled away at the remaining product liability litigation. Last quarter, it had reported 2,600 outstanding cases.
Most of the plaintiffs argued that they had been misled about the risks of diabetes and weight gain caused by Seroquel, the company’s second-best-selling product with $5.3 billion in worldwide sales last year, behind the cholesterol drug Crestor.
In the filing on Thursday, AstraZeneca said it added $55 million last quarter to the previously reported $592 million set-aside, for a total of $647 million to settle the litigation.
“Is it a good move?” said Les Funtleyder, health care analyst for Miller Tabak. “Yes, anytime you can settle these suits, it’s good.”
The set-aside includes the $68.5 million that the drug maker agreed to pay in March to 37 states, settling charges that it had illegally marketed Seroquel.
AstraZeneca also had to pay $520 million last year to settle federal investigations into its marketing of Seroquel.
At that time, Kathleen Sebelius, the secretary of health and human services, had accused the company of paying kickbacks to doctors while promoting the drug for unapproved uses by children, the elderly, veterans and prisoners.
AstraZeneca did not admit to any misconduct, while settling the federal case and signing a corporate integrity agreement, essentially putting it on probation with the government.
The company also said in the filing that its legal fees in Seroquel cases amounted to $743 million, partly covered by insurance. That figure has risen from $688 million estimated a year ago.
If the estimates hold, AstraZeneca will have paid a total of about $1.9 billion to defend and settle the personal injury cases and government investigations.
The figure represents less than five months of Seroquel sales.
That is far less than Eli Lilly paid to settle similar charges regarding its antipsychotic drug Zyprexa. Lilly paid more than $1.2 billion to settle lawsuits in 2007 and $1.4 billion to the government in 2009, including a criminal fine of $515 million.
At the time, that fine was the largest ever for a United States corporation. It has since eclipsed by the $1.3 billion criminal fine Pfizer paid as part of a $2.3 billion settlement of charges it had illegally marketed the painkiller Bextra and other drugs.
Drug makers have become the biggest targets of government antifraud investigations.
Legal discovery in the Seroquel cases has provided some of the most embarrassing or damaging disclosures over AstraZeneca’s past research and marketing practices, including a 1997 memo praising the company’s work to put a “positive spin” on a “cursed study” and highlighting one official who “has done a great ‘smoke-and-mirrors’ job!”
Another internal e-mail unsealed in court said AstraZeneca had “buried” unfavorable studies. A publications manager for the company wrote, “the larger issue is how we face the outside world when they begin to criticize us for suppressing data.”
http://prescriptions.blogs.nytimes.com/2011/07/28/astrazeneca-settles-most-seroquel-suits/








 And she was part of a team of people at
And she was part of a team of people at