Written By: 1 Boring Old Man
Posted on Tuesday 8 February 2011
As a practitioner I never used to pay a lot of attention to Clinical Trial data. I assumed that if the FDA approved a drug, it meant that it was effective and that it added something to the drug armamentarium we already had. Back in the days when I was actively involved with treating psychotic patients [Schizophrenia], we had a collection of anti-psychotic drugs [now called the First Generation Antipsychotics] that all worked, but had somewhat different side effect profiles. I used the ones that had the least side effects [but they all had them]. I guess the rule was to find the lowest dose of the one the patient was most likely to take. They’re hard drugs to use. There’s a constant worry about side effects [particularly the irreversible one - Tardive Dyskinesia] and the effect of "drugging" the patient’s mind. On the other side of that coin is a crippling psychosis that once sentenced patients to an "Institutionalized" Life. It’s very easy to get stuck on the evils of one or the other side of that problem – to argue about being able to live freely in society, or to see drugs as a toxic chemical straight-jacket. For some, one doesn’t have to struggle and things go smoothly. But for a significant number of people with that illness, there’s no "right course" and one has to live in that netherworld as a Psychiatrist, just as the afflicted live there themselves. I’d been there before with the sometimes toxic treatments used in severe physical diseases [those dire warnings they mumble at the end of the t.v. drug commercials], but somehow the conflict feels different in Psychiatry, but that’s another very long subject for another day.
 Right now, I want to focus on the Atypical Antipsychotic drug Seroquel, one that’s often in the news, and in the stories about the intrusion of the Pharmaceutical Industry into the practice of Medicine [Psychiatry] over the last twenty or thirty years. The Atypical Antipsychotics are a group of drugs that are chemically different from the older drugs. When they began to appear, they were touted as having less extrapyramidal side effects [so less chance of irreversible Tardive Dyskinesia] and a diminished side effect profile in general. I had little to no experience with the drugs in my practice as a psychoanalytic psychotherapist, but in retirement I volunteer in clinics where I see some psychotic patients and a whole lot of people who have been started on these drugs elsewhere. The thing of it is, most of the people I see on Seroquel aren’t psychotic – nor have they ever been. Since its FDA approval, it was later approved for Mania [another psychotic illness] and then even later as an add-on medication for people with Major Depressive Disorder [psychotic or not] that don’t respond to anti-depressants [SSRIs]. In practice, it seems to be being used for depressed people
Right now, I want to focus on the Atypical Antipsychotic drug Seroquel, one that’s often in the news, and in the stories about the intrusion of the Pharmaceutical Industry into the practice of Medicine [Psychiatry] over the last twenty or thirty years. The Atypical Antipsychotics are a group of drugs that are chemically different from the older drugs. When they began to appear, they were touted as having less extrapyramidal side effects [so less chance of irreversible Tardive Dyskinesia] and a diminished side effect profile in general. I had little to no experience with the drugs in my practice as a psychoanalytic psychotherapist, but in retirement I volunteer in clinics where I see some psychotic patients and a whole lot of people who have been started on these drugs elsewhere. The thing of it is, most of the people I see on Seroquel aren’t psychotic – nor have they ever been. Since its FDA approval, it was later approved for Mania [another psychotic illness] and then even later as an add-on medication for people with Major Depressive Disorder [psychotic or not] that don’t respond to anti-depressants [SSRIs]. In practice, it seems to be being used for depressed people 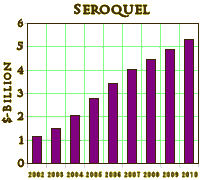 even if they don’t have "Major" depression, or people who have Anxiety, or people who have any number of other things including PTSD or insomnia of any kind. It’s being used a bit like the Benzodiazepines as an all purpose anxiolytic/sedative drug [we don't use the Benzodiazepines much anymore except short-term because they tend to be habituating and addictive]. The staggering point is that the Atypical Antipsychotics are the number one drugs prescribed in the U.S. right now [by cost]. And Seroquel is the number one in the class – to the tune of $4.9 Billion last year [2009]! Cough.
even if they don’t have "Major" depression, or people who have Anxiety, or people who have any number of other things including PTSD or insomnia of any kind. It’s being used a bit like the Benzodiazepines as an all purpose anxiolytic/sedative drug [we don't use the Benzodiazepines much anymore except short-term because they tend to be habituating and addictive]. The staggering point is that the Atypical Antipsychotics are the number one drugs prescribed in the U.S. right now [by cost]. And Seroquel is the number one in the class – to the tune of $4.9 Billion last year [2009]! Cough.If you’re reading this, you can’t have missed that AstraZeneca and its Seroquel have been on the radar of the increasing throng of people up in arms about Pharmaceutical Companies using Physicians in stealth advertising of their drugs, particularly in Psychiatry – paid Speaker’s Bureaus, Ghost-writing, Scientific Advisory Boards, Continuing Medical Education, unacknowledged Conflicts of Interest, direct relationships with Key Opinion Leaders and Academic Psychiatrists. In 2008, Senator Chuck Grassley’s investigations and the numerous suits against Pharmaceutical Companies belatedly put some of these practices on the front pages of our newspapers. I’ve been writing about these things a lot myself, including some things about Seroquel.
Recently, Stephany [soulful sepulcher] sent me the URL of a key to a sea of documents released in May 2009 from one of the cases against AstraZeneca [a suit about covering up the incidence of weight gain and Diabetes as a side effect]. I’d seen the document archive before, but was overwhelmed by the volume]. I’d failed to see the key that allows finding the various threads among the array of files [I really appreciate her sending it]. I’ve spent a few days perusing the memos and emails. It’s pretty incriminating stuff – people talking about how to hide or minimize negative studies, how to avoid mentioning side effects, having affairs with each other, how to hype off label uses, how to spin all sorts of things – incriminating and sometimes disgusting. As I’ve read through the evidence, I keep wondering how all this seedy business played out in getting the drug through the F.D.A. and on the market [and into such widespread and lucrative use].
What I’ve learned from the patients is that it’s a lightweight antipsychotic at best. The weight gain seems almost universal, and often prohibitive. When I ask people who are on it what it does for them, they sometimes say, "it calms me down," more usually say "It puts me to sleep," or even say "I can’t sleep without it." Depressed people on anti-depressants who have gotten it as an add-on say the same things. As a clinician, I’m underwhelmed. So instead of looking at those internal AstraZeneca documents or the suits against the company, I’ve decided I want to go back and look at the beginning – how it got approved. I’m not the first person to walk this road, but I want to do it myself as a way of getting into this Clinical Trial world I talked about in that evidence-based medicine series I meandered through recently. It’s a long, cold, winter here in the Georgia mountains, and this is as good a hobby as any until the place thaws out and I can get back to communing with whatever old people commune with when they move to the woods. So the question, "How did Seroquel, a softy with a big downside, get approved in the first place? for so many things? and get to be such a financial blockbuster?" Maybe after looking at those Clinical Trials that got Seroquel approved, I’ll reference some of those juicy, unsavory documents in that document archive…
http://1boringoldman.com/index.php/2011/02/08/seroquel-i-introduction-of-an-atypical/
No comments:
Post a Comment