Written By: 1 Boring Old Man
Posted on Thursday 10 February 2011

 Moving along. Trial 0008 was Zeneca‘s most successful trial for Seroquel, published in a more widely distributed journal. I’ve included the abstract in part because it describes the actual dosing schedule for Seroquel omitted from the F.D.A. statistical report.
Moving along. Trial 0008 was Zeneca‘s most successful trial for Seroquel, published in a more widely distributed journal. I’ve included the abstract in part because it describes the actual dosing schedule for Seroquel omitted from the F.D.A. statistical report. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo
Archives of General Psychiatry. 1997 Jun;54(6):549-57.
by Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CG.
Division of Mental Health, Larue D. Carter Memorial Hospital, Indianapolis, Ind.
AbstractBACKGROUND: Quetiapine fumarate (Seroquel [ICI 204,636]) is an atypical dibenzothiazepine antipsychotic with a greater affinity for 5-hydroxytryptamine2 (5-HT2) receptors than for D2 dopamine receptors; its efficacy in patients with schizophrenia was shown in early phase 2 trials (maximum dose, 750 mg/d).METHODS: In this multicenter, double-blind, placebo-controlled trial, 286 patients hospitalized with chronic or subchronic schizophrenia [DSM-III-R] were randomized to 6 weeks of treatment with high-dose quetiapine fumarate (< or = 750 mg/d), n = 96; low-dose quetiapine fumarate (< or = 250 mg/d), n = 94; or placebo, n = 96. The Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression Severity of Illness item scores were the primary efficacy variables. Secondary efficacy variables included the BPRS positive-symptom cluster score, the Modified Scale for the Assessment of Negative Symptoms summary score (United States only), and the total score from the negative scale of the Positive and Negative Syndrome Scale (Europe only). Scores were analyzed using an analysis of covariance for change from baseline at end point with last observations carried forward. The model included baseline score (covariate), center, and treatment. Extrapyramidal symptoms were assessed using the Simpson-Angus Scale and the Barnes Akathisia Scale; abnormal involuntary movements were assessed using the Abnormal Involuntary Movement Scale. Frequency distributions of grouped change-from-baseline scores were analyzed using chi 2 tests.RESULTS: Of 280 patients in whom the efficacy of quetiapine was evaluated, 159 (42% of those receiving high-dose treatment; 57%, low-dose treatment; and 59%, placebo) withdrew before trial completion, primarily because of treatment failure. Significant (P < .001, BPRS; P = .003, Clinical Global Impression Severity of Illness item; and P = .003, BPRS positive-symptom cluster) differences were identified between patients receiving high-dose quetiapine and placebo for both primary efficacy variables, with end point differences in the BPRS positive-symptom cluster score showing quetiapine’s consistency in reducing positive symptoms. The reduction of negative symptoms was less consistent; high-dose quetiapine was superior on the Modified Scale for the Assessment of Negative Symptoms but not on the negative scale of the Positive and Negative Syndrome Scale. Quetiapine was well tolerated and did not induce extrapyramidal symptoms, sustained elevations of prolactin, or clinically significant changes in hematologic parameters.CONCLUSIONS: Quetiapine is an effective antipsychotic with a favorable safety profile. The optimum dose is probably greater than 250 mg/d.
Here’s what the report said, followed by some of the data from Trial 0008:
"This 6-week study randomized a total of 286 patients among 37 centers in the US (59%) and Europe (41 %): N=96 High dose, N=94 Low dose, N=96 Placebo… A total of 4 patients had no post-baseline data, so that 282 patients comprise the effcacy ITT population. The endpoints of interest are BPRS total, key BPRS, CGI Severity and the negative PANSS (only in Europe) and the SANS (done only in the US). The protocol states that ANCOVA with baseline as the covariate would be used for all analyses. However, there is no plan for multiple comparisons among treatment arms. Table 1 displays the baseline conditions and demographics of the treatment groups. Completion rates were 50% for High dose, 43% for Low dose, and 41% for Placebo. ‘Treatment failure’ accounted for between 40%-50% of the dropouts."
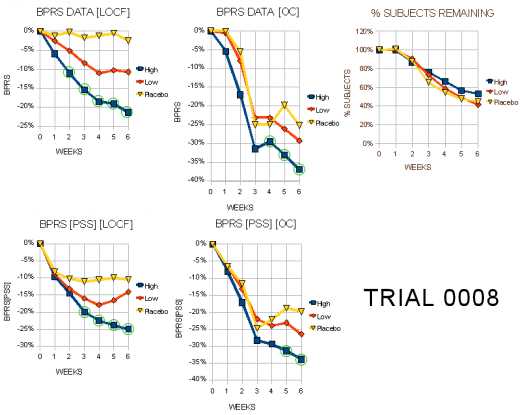

 I expect the people at Zeneca were pretty pleased to see those LOCF graphs on the left. That’s exactly what they hoped for. Even the OC graphs have the green circles indicating statistical significance [though they're not quite so pretty]. Right now, we’re not talking about relevance so I’ll reserve any of those comments for a later time. Again, there were a lot of drop-outs. I’ve attempted to roughly translate the awkward bar graphs of the scores for the subjects that dropped out into a more readable form [the graph on the right is the inverse of the one above]:
I expect the people at Zeneca were pretty pleased to see those LOCF graphs on the left. That’s exactly what they hoped for. Even the OC graphs have the green circles indicating statistical significance [though they're not quite so pretty]. Right now, we’re not talking about relevance so I’ll reserve any of those comments for a later time. Again, there were a lot of drop-outs. I’ve attempted to roughly translate the awkward bar graphs of the scores for the subjects that dropped out into a more readable form [the graph on the right is the inverse of the one above]: 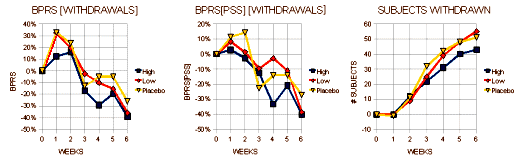
As in Trial 0006, the early Trial 0008 patients who dropped out were getting worse – more in the Placebo and Low Dose ["< or = 250 mg/d"] groups, but it wasn’t enough to obfuscate the differences they were looking for [as always there are questions - why did so many who were decidedly better drop out at the end?]. Here’s what the reviewers said about Trial 0008:
"Tables 3a and 3b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. It is not surprising that the high dose is statistically significant but the low dose is not, since the trial was planned (power= 90%) to find a 9 point difference between the high dose and placebo arms for the BPRS… Tables 5a and 5b display the results of the key positive symptom BPRS cluster for the LOCF and observed cases, respectively."
I wanted to look at the full text of the published article to see how they presented the data [and to see who was in the Acknowledgements - "medical writing"] but the download creeped along through an afternoon nap, some of the Egyptian revolution, and Mubarak’s interminable [and disappointing] speech. So I decided to let it run overnight during "off-hours." I’ll post that part later as an update.
For the moment I’ll say that this study does seem to show Seroquel to be an antipsychotic drug – nothing to set off fireworks or to write home about, but an antipsychotic drug nonetheless. As for the Clinical Trials world itself, it’s not a direct learning lab for the practice of clinical medicine. In this study, it took three or four weeks to separate itself from placebos. But the two findings that actually impressed me in this and the last study were:
- In the first few weeks, Seroquel appeared to allow the subjects to stay in the study, suggesting that it was helpful early on.
- The High Dose Seroquel group had a 9% lower drop-out rate overall, again suggesting it was helpful.
The idea of "keeping people on the medication" as an index of success will play an increasingly prominent role in later studies of efficacy, but for the moment, Zeneca put a point on their side of the scoreboard for Seroquel approval with Trial 0008.
Update [at 1:00 AM it downloaded immediately]:
Well, to the Acknowledgements:
Accepted for publication November 6, 1996. This study was supported by a grant from Zeneca Pharmaceuticals, Macclesfield, England, and Wilmington, Del. We thank Suzanne Bristow-Marcalus for medical writing support; Jean Fennimore, RN, and Alison Smith, MSc, for trial organization and management support; Gary Cooper, MS, Jacqueline Fiore, Lesley Farrow, and Barney Home for trial monitoring support; and Kathleen Jelliffe, Denise Redkar-Brown, Joy Russo, and Thais Womack for data management support.
By today’s standards, that’s suspect for ghost-writing. I think we can safely assume that this article was written in Delaware at Zeneca‘s headquarters.
http://1boringoldman.com/index.php/2011/02/10/seroquel-iii-their-best-shot%E2%80%A6/
No comments:
Post a Comment